 Found 152 hits of affinity data for UniProtKB/TrEMBL: Q9H3Y6
Found 152 hits of affinity data for UniProtKB/TrEMBL: Q9H3Y6 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM255303
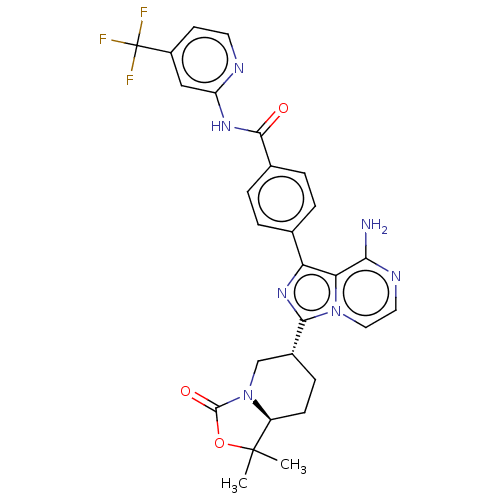
(US9481682, 52)Show SMILES CC1(C)OC(=O)N2C[C@@H](CC[C@@H]12)c1nc(-c2ccc(cc2)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C28H26F3N7O3/c1-27(2)19-8-7-17(14-38(19)26(40)41-27)24-36-21(22-23(32)34-11-12-37(22)24)15-3-5-16(6-4-15)25(39)35-20-13-18(9-10-33-20)28(29,30)31/h3-6,9-13,17,19H,7-8,14H2,1-2H3,(H2,32,34)(H,33,35,39)/t17-,19+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRMS (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM255256
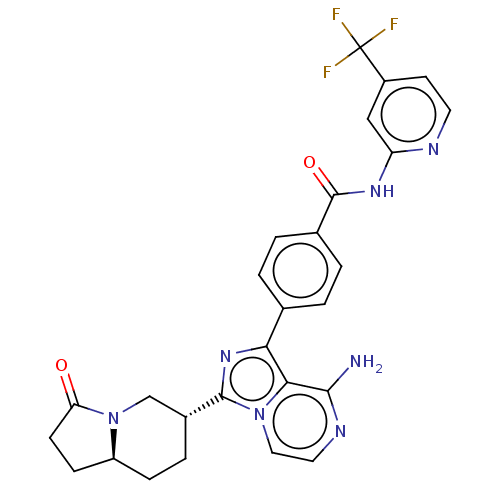
(US9481682, 4)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CCC(=O)N2C1 |r| Show InChI InChI=1S/C27H24F3N7O2/c28-27(29,30)18-9-10-32-20(13-18)34-26(39)16-3-1-15(2-4-16)22-23-24(31)33-11-12-36(23)25(35-22)17-5-6-19-7-8-21(38)37(19)14-17/h1-4,9-13,17,19H,5-8,14H2,(H2,31,33)(H,32,34,39)/t17-,19+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRMS (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50560327
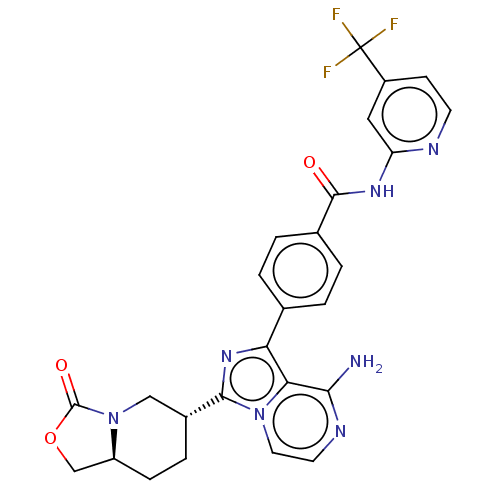
(CHEMBL4749037)Show SMILES [H][C@]12COC(=O)N1C[C@@H](CC2)c1nc(-c2ccc(cc2)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRMS (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM255338

(US9481682, 116 | US9481682, 88)Show SMILES COc1cc(ccc1-c1nc([C@@H]2CC[C@H]3CCC(=O)N3C2)n2ccnc(N)c12)C(=O)Nc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C28H26F3N7O3/c1-41-20-12-15(27(40)35-21-13-17(8-9-33-21)28(29,30)31)3-6-19(20)23-24-25(32)34-10-11-37(24)26(36-23)16-2-4-18-5-7-22(39)38(18)14-16/h3,6,8-13,16,18H,2,4-5,7,14H2,1H3,(H2,32,34)(H,33,35,40)/t16-,18+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRMS (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM267959
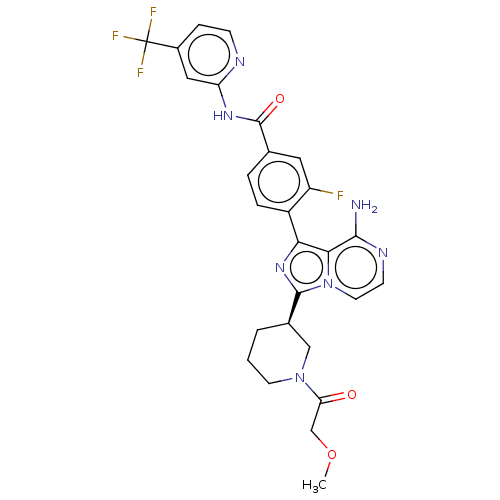
(4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...)Show SMILES COCC(=O)N1CCC[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C27H25F4N7O3/c1-41-14-21(39)37-9-2-3-16(13-37)25-36-22(23-24(32)34-8-10-38(23)25)18-5-4-15(11-19(18)28)26(40)35-20-12-17(6-7-33-20)27(29,30)31/h4-8,10-12,16H,2-3,9,13-14H2,1H3,(H2,32,34)(H,33,35,40)/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM267790
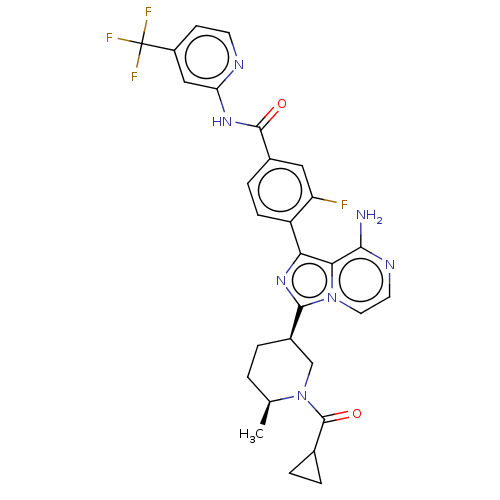
(4-(8-amino-3-{(3R,6S)-6-methyl-1-[(3-methyloxetan-...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C1CC1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C29H27F4N7O2/c1-15-2-3-18(14-40(15)28(42)16-4-5-16)26-38-23(24-25(34)36-10-11-39(24)26)20-7-6-17(12-21(20)30)27(41)37-22-13-19(8-9-35-22)29(31,32)33/h6-13,15-16,18H,2-5,14H2,1H3,(H2,34,36)(H,35,37,41)/t15-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SRMS |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00463
BindingDB Entry DOI: 10.7270/Q25B065B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50558112
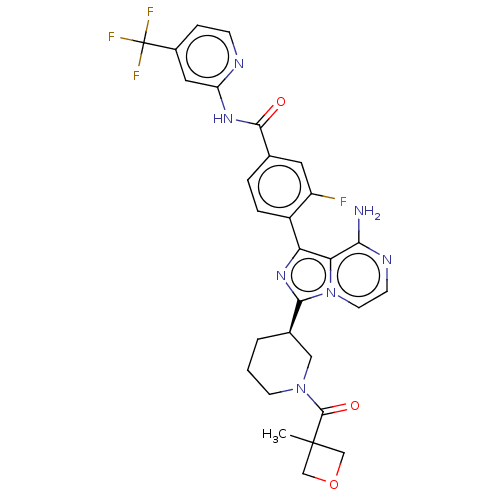
(CHEMBL4799627)Show SMILES CC1(COC1)C(=O)N1CCC[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SRMS |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00463
BindingDB Entry DOI: 10.7270/Q25B065B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM267703
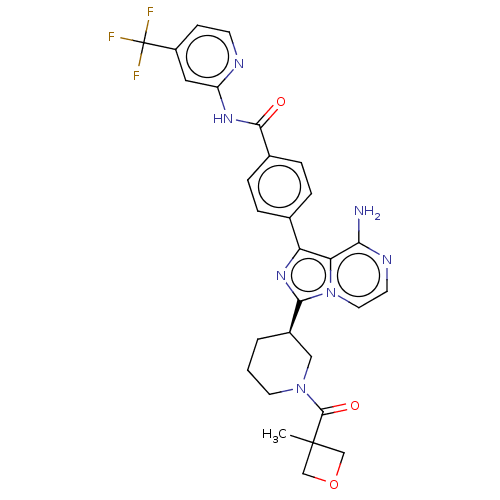
((R)-4-(8-amino-5-deutero-3-(1-(3-methoxypropanoyl)...)Show SMILES CC1(COC1)C(=O)N1CCC[C@H](C1)c1nc(-c2ccc(cc2)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C29H28F3N7O3/c1-28(15-42-16-28)27(41)38-11-2-3-19(14-38)25-37-22(23-24(33)35-10-12-39(23)25)17-4-6-18(7-5-17)26(40)36-21-13-20(8-9-34-21)29(30,31)32/h4-10,12-13,19H,2-3,11,14-16H2,1H3,(H2,33,35)(H,34,36,40)/t19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SRMS |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00463
BindingDB Entry DOI: 10.7270/Q25B065B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50269558
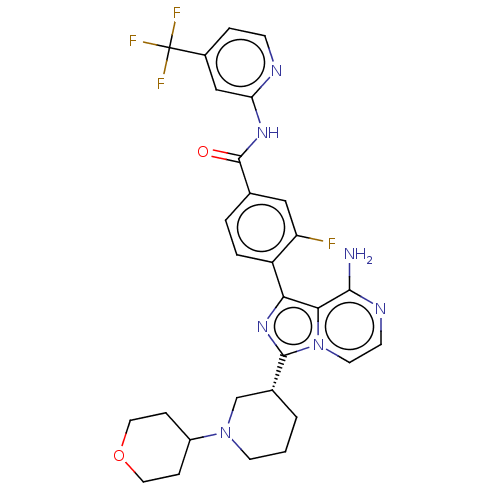
(CHEMBL4077588)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3F)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CCCN(C1)C1CCOCC1 |r| Show InChI InChI=1S/C29H29F4N7O2/c30-22-14-17(28(41)37-23-15-19(5-8-35-23)29(31,32)33)3-4-21(22)24-25-26(34)36-9-11-40(25)27(38-24)18-2-1-10-39(16-18)20-6-12-42-13-7-20/h3-5,8-9,11,14-15,18,20H,1-2,6-7,10,12-13,16H2,(H2,34,36)(H,35,37,41)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM255370

(US9481682, 121)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3OC3CC3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CCC(=O)N2C1 |r| Show InChI InChI=1S/C30H28F3N7O3/c31-30(32,33)18-9-10-35-23(14-18)37-29(42)16-2-7-21(22(13-16)43-20-5-6-20)25-26-27(34)36-11-12-39(26)28(38-25)17-1-3-19-4-8-24(41)40(19)15-17/h2,7,9-14,17,19-20H,1,3-6,8,15H2,(H2,34,36)(H,35,37,42)/t17-,19+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRMS (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50269555
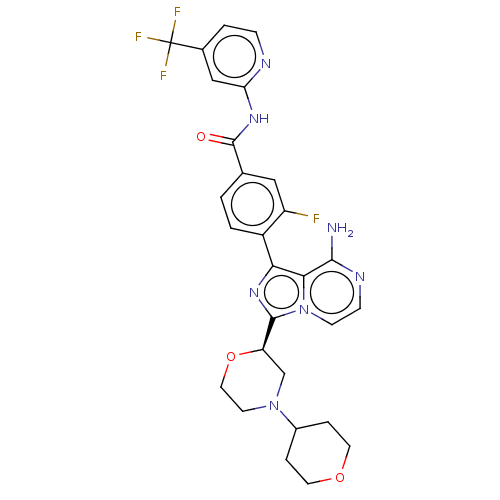
(CHEMBL4060757)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3F)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@H]1CN(CCO1)C1CCOCC1 |r| Show InChI InChI=1S/C28H27F4N7O3/c29-20-13-16(27(40)36-22-14-17(3-6-34-22)28(30,31)32)1-2-19(20)23-24-25(33)35-7-8-39(24)26(37-23)21-15-38(9-12-42-21)18-4-10-41-11-5-18/h1-3,6-8,13-14,18,21H,4-5,9-12,15H2,(H2,33,35)(H,34,36,40)/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM267935
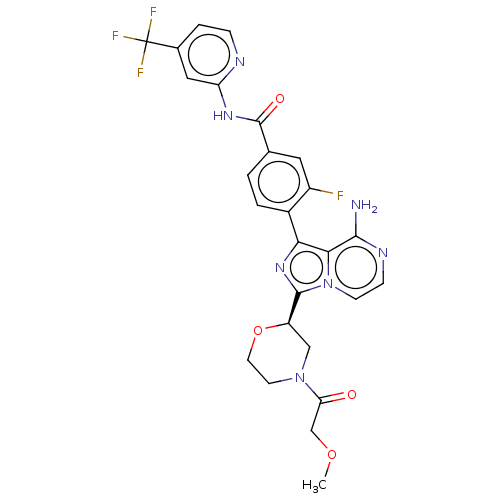
(4-{8-amino-3-[(2R)-4-oxetan-3-ylmorpholin-2-yl]imi...)Show SMILES COCC(=O)N1CCO[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C26H23F4N7O4/c1-40-13-20(38)36-8-9-41-18(12-36)24-35-21(22-23(31)33-6-7-37(22)24)16-3-2-14(10-17(16)27)25(39)34-19-11-15(4-5-32-19)26(28,29)30/h2-7,10-11,18H,8-9,12-13H2,1H3,(H2,31,33)(H,32,34,39)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50269557

(CHEMBL4069387)Show SMILES CC(C)C(=O)N1CCC[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C28H27F4N7O2/c1-15(2)27(41)38-10-3-4-17(14-38)25-37-22(23-24(33)35-9-11-39(23)25)19-6-5-16(12-20(19)29)26(40)36-21-13-18(7-8-34-21)28(30,31)32/h5-9,11-13,15,17H,3-4,10,14H2,1-2H3,(H2,33,35)(H,34,36,40)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50269556
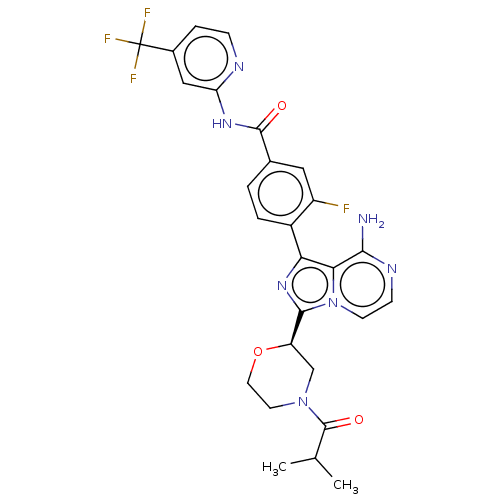
(CHEMBL4075954)Show SMILES CC(C)C(=O)N1CCO[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C27H25F4N7O3/c1-14(2)26(40)37-9-10-41-19(13-37)24-36-21(22-23(32)34-7-8-38(22)24)17-4-3-15(11-18(17)28)25(39)35-20-12-16(5-6-33-20)27(29,30)31/h3-8,11-12,14,19H,9-10,13H2,1-2H3,(H2,32,34)(H,33,35,39)/t19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50577406
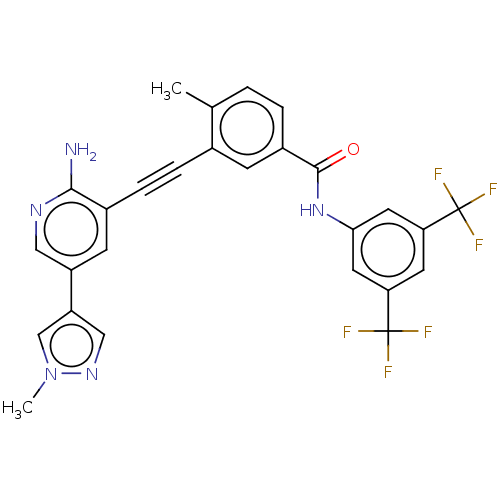
(CHEMBL4862806)Show SMILES Cc1ccc(cc1C#Cc1cc(cnc1N)-c1cnn(C)c1)C(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SRMS by radiometric scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00976
BindingDB Entry DOI: 10.7270/Q2J96B6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50506460

(CHEMBL4467948)Show SMILES Cc1ccc(cc1C#Cc1cc(cnc1N)-c1cnn(C)c1)C(=O)Nc1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H19Cl2N5O/c1-15-3-4-18(25(33)31-23-8-7-21(26)11-22(23)27)9-16(15)5-6-17-10-19(12-29-24(17)28)20-13-30-32(2)14-20/h3-4,7-14H,1-2H3,(H2,28,29)(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SRMS by radiometric scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00976
BindingDB Entry DOI: 10.7270/Q2J96B6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50613392
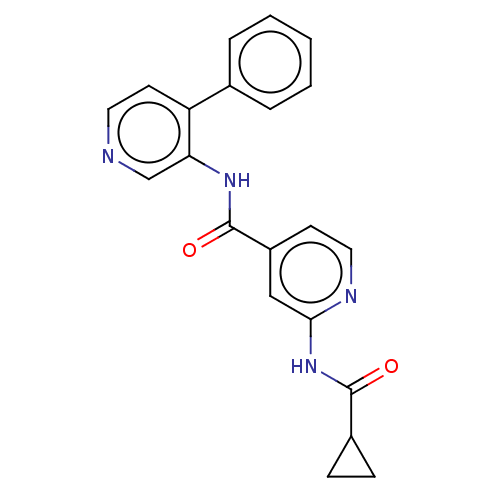
(CHEMBL5270581)Show SMILES O=C(Nc1cc(ccn1)C(=O)Nc1cnccc1-c1ccccc1)C1CC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50613393
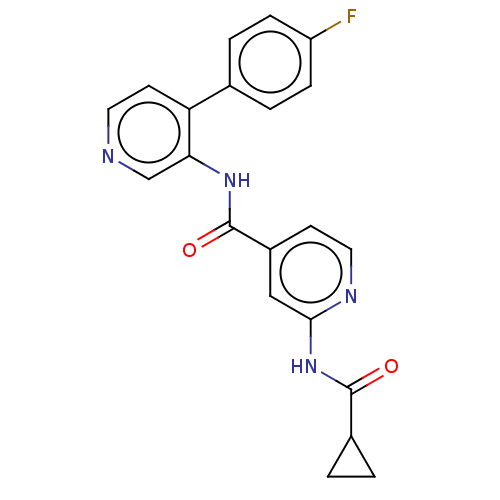
(CHEMBL5288565)Show SMILES Fc1ccc(cc1)-c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50613394
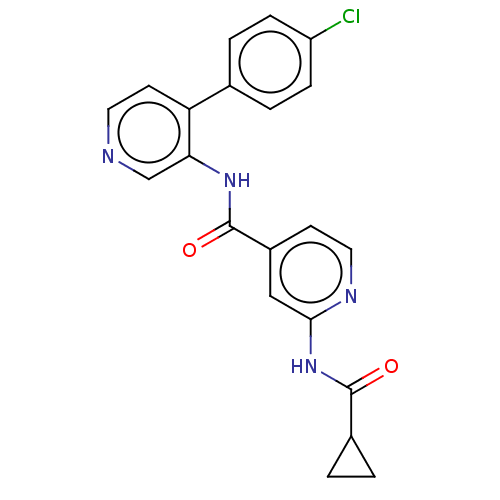
(CHEMBL5278465)Show SMILES Clc1ccc(cc1)-c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SRMS using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human SRMS using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50499634
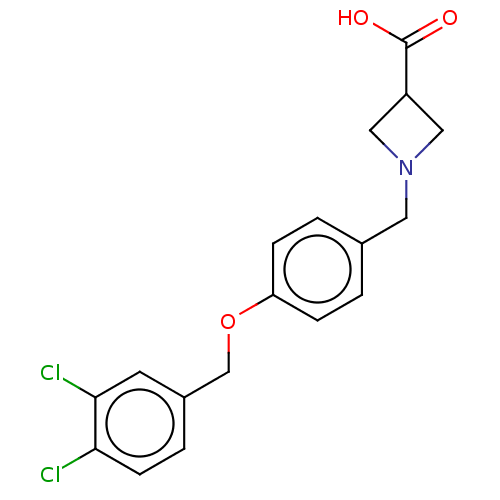
(CHEMBL3741589)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3ccc(Cl)c(Cl)c3)cc2)C1 Show InChI InChI=1S/C18H17Cl2NO3/c19-16-6-3-13(7-17(16)20)11-24-15-4-1-12(2-5-15)8-21-9-14(10-21)18(22)23/h1-7,14H,8-11H2,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of SRMS (unknown origin) |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human SRMS using poly[Glu:Tyr] (4:1) as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50537742
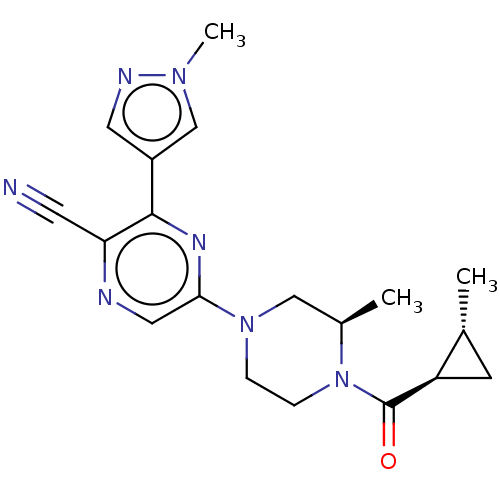
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human GST-tagged SRM expressed in baculovirus expression system using tyrosine-1 peptide as substrate incubated... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM31085
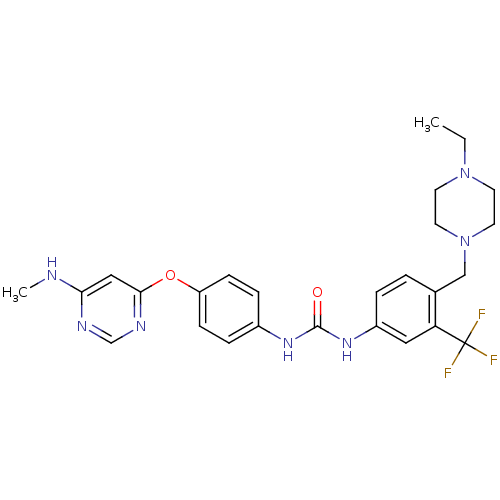
(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM26300
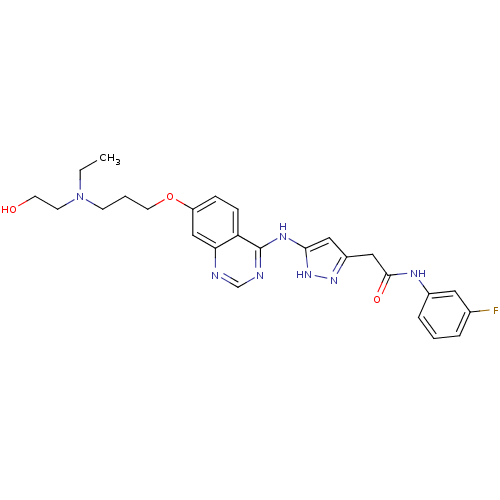
(2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}qu...)Show SMILES CCN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1 Show InChI InChI=1S/C26H30FN7O3/c1-2-34(10-11-35)9-4-12-37-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(36)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17,35H,2,4,9-12,15H2,1H3,(H,30,36)(H2,28,29,31,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM31099
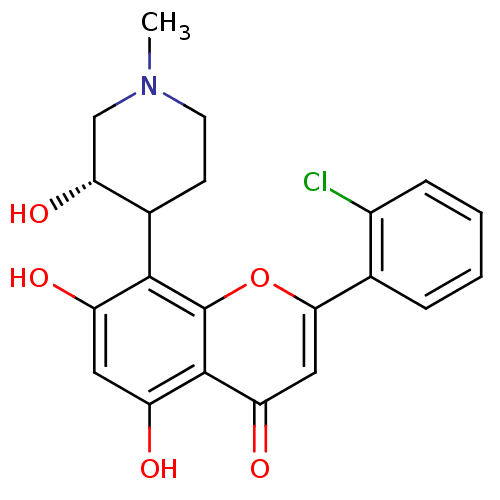
(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S)-3-hydroxy...)Show SMILES CN1CCC([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM26474
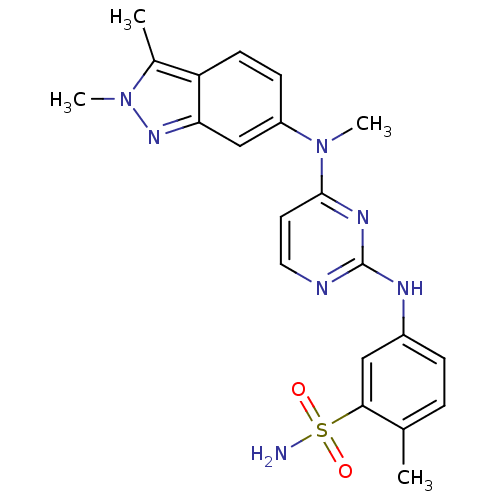
(5-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...)Show SMILES CN(c1ccc2c(C)n(C)nc2c1)c1ccnc(Nc2ccc(C)c(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C21H23N7O2S/c1-13-5-6-15(11-19(13)31(22,29)30)24-21-23-10-9-20(25-21)27(3)16-7-8-17-14(2)28(4)26-18(17)12-16/h5-12H,1-4H3,(H2,22,29,30)(H,23,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM31095
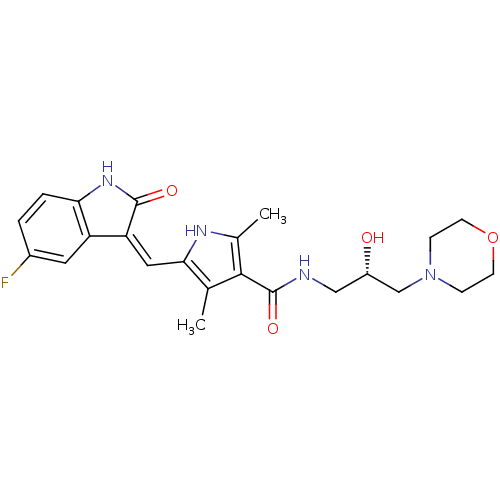
(5-[(Z)-(5-fluoranyl-2-oxidanylidene-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NC[C@H](O)CN1CCOCC1 Show InChI InChI=1S/C23H27FN4O4/c1-13-20(10-18-17-9-15(24)3-4-19(17)27-22(18)30)26-14(2)21(13)23(31)25-11-16(29)12-28-5-7-32-8-6-28/h3-4,9-10,16,26,29H,5-8,11-12H2,1-2H3,(H,25,31)(H,27,30)/b18-10-/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM31096

(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM21
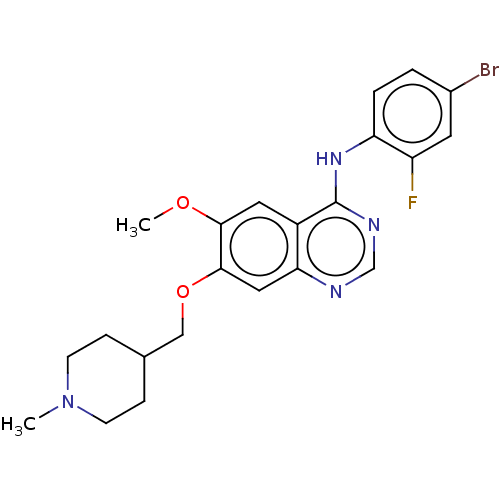
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VD6WVR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50308060
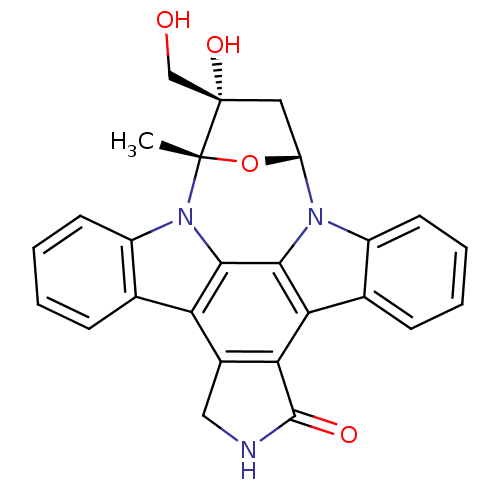
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50326053
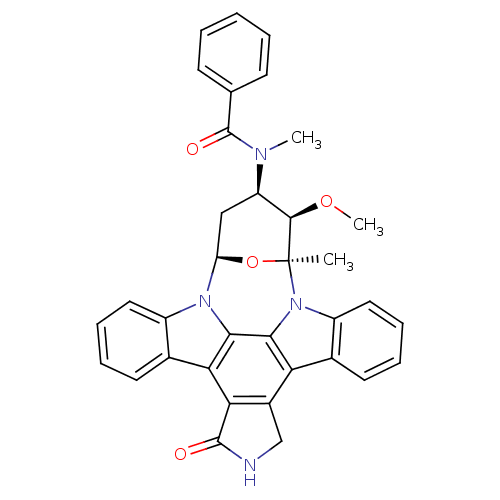
(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50026612

(BIBF-1120 | Nintedanib | US10981896, Compound Nint...)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM50326054
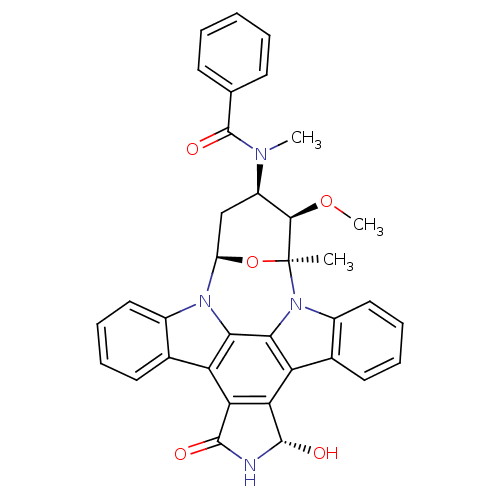
(CHEMBL1240703)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4[C@H](O)NC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O5/c1-35-31(43-3)23(37(2)34(42)18-11-5-4-6-12-18)17-24(44-35)38-21-15-9-7-13-19(21)25-27-28(33(41)36-32(27)40)26-20-14-8-10-16-22(20)39(35)30(26)29(25)38/h4-16,23-24,31,33,41H,17H2,1-3H3,(H,36,40)/t23-,24-,31-,33+,35+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM13535
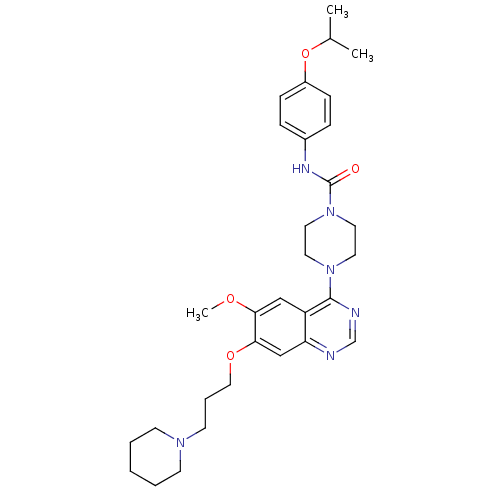
(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to SRMS |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM5655

(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...)Show SMILES CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM31095

(5-[(Z)-(5-fluoranyl-2-oxidanylidene-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NC[C@H](O)CN1CCOCC1 Show InChI InChI=1S/C23H27FN4O4/c1-13-20(10-18-17-9-15(24)3-4-19(17)27-22(18)30)26-14(2)21(13)23(31)25-11-16(29)12-28-5-7-32-8-6-28/h3-4,9-10,16,26,29H,5-8,11-12H2,1-2H3,(H,25,31)(H,27,30)/b18-10-/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Srms
(Homo sapiens (Human)) | BDBM25121

(4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C28H39N7O3/c1-5-22-27(37)34(3)23-17-29-28(32-25(23)35(22)20-8-6-7-9-20)31-21-11-10-18(16-24(21)38-4)26(36)30-19-12-14-33(2)15-13-19/h10-11,16-17,19-20,22H,5-9,12-15H2,1-4H3,(H,30,36)(H,29,31,32)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for SRMS kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data